The Kreb’s cycle/TCA cycle/Citric acid cycle is a cornerstone of most introductory biology discussions of energetics. Boy is it scary to look at, but what a wonderful opportunity to make students memorize names and structures! But seriously… short of asking students to know everything about everything, what are the core concepts that they ‘ought’ to understand?
To my mind, two words: energetic electrons
The TCA cycle is all about taking ‘energy’ out of glucose and somehow prepping ‘energy’ for processing by the mitochondrion. What is this ‘energy’? I think this has to be the focus of these discussions: in what form is the energy, how is it contained, and how is it transported. In sugar, we’re clearly talking about electrons, or more precisely, the state of electrons.
I like to do a fun little exercise with students asking them to characterize CH4 (methane; natural gas) and CH3CH2CH2CH2CH2CH2CH2CH3 (octane) vs. H2O and CO2. With some prodding, we get:
Behaviors: the first two can be used in fire & combustion; the second two extinguish fires
Chemistry: the first two contain C-H and C-C bonds; the latter two contain only C-O and O-H bonds
Conclusion: there is something ‘exciting’ about (some) equally shared electrons (C-C, C-H) and something dull about electrons that are tightly held by one partner (oxygen in these examples).
Thus, the ‘source’ in sugar is those electrons in C-H or C-C bonds. We need to get ’em out of there.
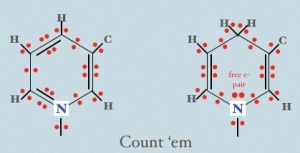
Here’s where it gets really, really weird. The carefully wrought, highly detailed figure from Wikipedia is a great example: great detail on names of enzymes, structures of molecules, players, etc. But you know what you never see? (and don’t blame Wiki; I have yet to see this in a single textbook): electrons! This is really weird–the entire point of the TCA cycle is to extract energetic electrons and we never talk about the poor fellas. How we represent things communicates to students what the figure is ‘for’; what they should be learning. The electrons should be bright blue, flashy and they should be the things to which the eye is drawn. The attached figure duos have an exciting ATP and GTP, but for the most part, I notice the oxygens… which per the reasoning above, are the dullest part (or rather, their electrons are).
My view: we must follow the electrons. What’s more, it’s mostly fun and easy detective work. Every time you see an NADH + H+ peel off if you look carefully at the before and after structures you can see that an electron pair [a covalent bond] has gone missing. Check me on this! What’s more, if we carefully examine the structures of NAD+ and NADH (below), we can verify that the two differ primarily by 2 electrons (red)… despite the fact that if you just memorize their names, there is no mention of the precious cargo whatsoever.
Summary: as with so many things, what is important (what form the ‘energy’ is in, where it goes) is hard to test. How many ways can you ask a student to tell you “It’s the electrons, stupid”. But surely our desire to write unambiguous multiple choice exams cannot be the primary driver of our teaching.
At some point, I’ll get into other big ideas in biological redox, notably that the potential energy of the electrons must be PRESERVED as they are ‘carried’ by NAD. As I often put it–it’s not whether you have a bucket of water, it’s how HIGH that bucket is.

Pingback: Molecule display in introductory biology