While primarily claimed by chemistry (and rightly so, I grudgingly admit), the periodic table is a delightfully clear example of DISCOVERY science and of fundamental importance to an understanding of Biology. I’m creating a distinction here between what I might call ‘fill in the gaps’ science or ‘next steps at the endpoints’ science because I think students are exposed almost exclusively to the latter two. In this usage, discovery science would be discerning a new law or pattern in nature, establishing an ordering principle, or revealing something transcendent. Many of these situations arise because of recognition of ordering patterns; the periodic table is perhaps one of the most clear and readily understood.
There are so many important patterns in the periodic table that even today it exists in many forms. It’s valuable to understand (and share with students) where it came from. In some ways, it’s one of those discoveries whose time had come–there were several universally recognized sub-patterns: the triads (columns with three members known at that time), the ‘law of octaves’ (since the entire noble gas column was ‘missing’, every eighth element
 (source)
(source)
among the lighter elements was a repeat, leading to some delightful thinking about correspondence to musical scales). Both these are nicely explained in this short history.
There are several aspects that set this ‘type’ of science apart from others. In its initial form, the Periodic Table did not add ‘facts’ to our collection–it added an organizing way of looking at facts-in-existence. It was a discovery that made a clutter of information snap into place. In many ways, then, it was bigger than measurements and counting in that it shrank the universe of scientific knowledge–by understanding the RULES of the table, we needed to ‘memorize’ fewer details from within. Lest I become too focused on what it did with existing information, it should immediately and importantly be noted that gaps in the table made for predictions about facts-to-be-found, whose confirmation represented solidification of the tables bona fides.
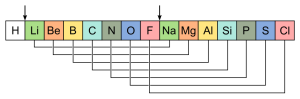
One of the aspects about the Periodic Table that I find fascinating is what it was whispering to us about things we weren’t ready to investigate in detail. The common behaviors that lead to the organization of the columns reflected how close orbitals were to filling with electrons–neither of which were known at the time. While the absence of the noble gases lead to a premature ‘ count’, the early structure of the table nonetheless was speaking about ‘completion’–the fact that the ROWS repeated after (7 then; 8 now) units meant that something ‘finished’ at eight, which we now know to be the filling of an outer shell. We can extend this analysis further, too: it was known that halogens (Cl, Br, F) combined with metals (K, Na) to create salts–there was something complementary/interactive about these players that were on respective ‘edges’ of the table. What is that thing? The path to a full outer shell. The metals had an ‘extra’ electron whose removal revealed the previous shell; the halogens needed only one addition to complete theirs. There was similar information about ‘combinatorics’–ratios of different elements in forming compounds with oxygens again arises from the outer shell electron count.
There are many, many other examples of patterns in scientific discovery, of course. The structure of DNA held within it the discovery of how replication must occur, how meaning was ‘held’ in molecules. The first useful information about cholera and hand washing by physicians arose through similar means. I think it’s important that we explicitly expose students to this kind of science–and potentially engaging and exciting as well.