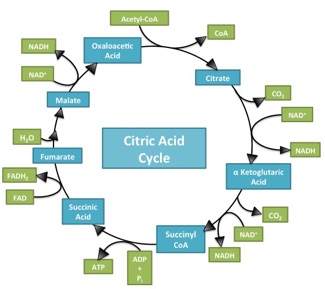
What makes the Citric Acid Cycle (Kreb’s cycle, TCA cycle) ‘hard’? Misdirection. When most of us sit down to learn something, the first question is usually “what is important here”. When trying to figure out a complicated process (Americans: think about the sport of cricket!), the questions are “What should I be looking at? And what am I seeing when I look there?”. It’s deeply ironic, then, that in looking at Introductory Biology textbooks, it’s incredibly difficult to spot an electron anywhere in representations of the citric acid cycle. This is bizarre, in that the cycle has only two jobs: 1) extract ‘high value’ (energetic) electrons, and 2) be a cycle so it can keep… cycling. A call for electron-watching and some suggestions for teaching electron flow through the citric acid cycle follow (Image source) Continue reading
 Both studies and common sense indicate that common threads running through our teaching provide students with reinforcement of both thread components and the things they connect. This approach also highlights conveying principles rather than fact collecting as our learning objective. The details of a basic ATP hydrolysis reaction illustrate both key principles (how enzymes actually implement their abstract aspects [speed up reaction; lower reaction barrier], roles for specific amino acids and protein folding), value of understanding chemistry in thinking about biology) as well as providing students with a tool that they’ll see over and over… and over again: the core mechanism is found in nucleotide addition, kinase and phosphatase reactions, pre-mRNA splicing, the timing mechanisms of GTPases [tubulin, EF-Tu, GPCRs], and… oh yeah: virtually every ATP-driven or -coupled reaction in the cell!
Both studies and common sense indicate that common threads running through our teaching provide students with reinforcement of both thread components and the things they connect. This approach also highlights conveying principles rather than fact collecting as our learning objective. The details of a basic ATP hydrolysis reaction illustrate both key principles (how enzymes actually implement their abstract aspects [speed up reaction; lower reaction barrier], roles for specific amino acids and protein folding), value of understanding chemistry in thinking about biology) as well as providing students with a tool that they’ll see over and over… and over again: the core mechanism is found in nucleotide addition, kinase and phosphatase reactions, pre-mRNA splicing, the timing mechanisms of GTPases [tubulin, EF-Tu, GPCRs], and… oh yeah: virtually every ATP-driven or -coupled reaction in the cell!